In the booming health and wellness industry, bioactive peptides have gained growing attention for their scientifically validated benefits. From collagen supplements to functional sports nutrition, these peptides serve a variety of physiological roles. But not all peptides are created equal — their size significantly impacts their absorption, bioavailability, and biological effectiveness. This article explores the science behind small-molecule peptides, explains why size matters, and showcases common examples used in cutting-edge functional nutrition products.
I. Introduction: The Rise of Small-Molecule Peptides in Functional Nutrition
Peptides, short chains of amino acids, play essential roles in cellular signaling, tissue regeneration, immunity, and metabolism. In recent years, the development of enzymatically hydrolyzed protein products has enabled the production of small-molecule peptides — typically under 1000 Daltons (Da) in molecular weight [1].
These peptides have emerged as key ingredients in dietary supplements, beauty-from-within products, anti-aging solutions, sports nutrition formulas, and even therapeutic foods. Why are these small molecules so effective? The answer lies in their size.
II. What Are Small-Molecule Peptides?
Small-molecule peptides generally refer to dipeptides (2 amino acids), tripeptides (3 amino acids), and oligopeptides composed of fewer than 10 amino acids, with a molecular weight typically under 1000 Da. Their compact size offers distinct advantages:
- Enhanced intestinal absorption: Smaller peptides can be absorbed directly through peptide transporters in the gut wall [2].
- High bioavailability: They quickly enter the bloodstream and reach target tissues.
- Functional specificity: Many small peptides have well-defined bioactivities, such as antioxidant, anti-inflammatory, or ACE-inhibitory effects [3].
In contrast, large peptides or intact proteins must be further broken down by digestive enzymes, which may limit their bioefficacy.
III. Why Molecular Size Matters: The Science of Absorption and Efficacy
The body’s ability to absorb peptides is tightly linked to their molecular weight. Peptide transporters in the intestinal epithelium — mainly PEPT1 — are designed to recognize di- and tri-peptides, making small-molecule peptides more efficiently absorbed than larger ones [2].
Smaller peptides also bypass some digestive degradation, enter circulation faster, and exhibit more targeted functionality. These qualities make them ideal for applications where rapid action or high absorption is essential — such as in skin health, athletic recovery, metabolic support, and immune modulation.
In short: size is not just a characteristic — it’s a functional advantage.
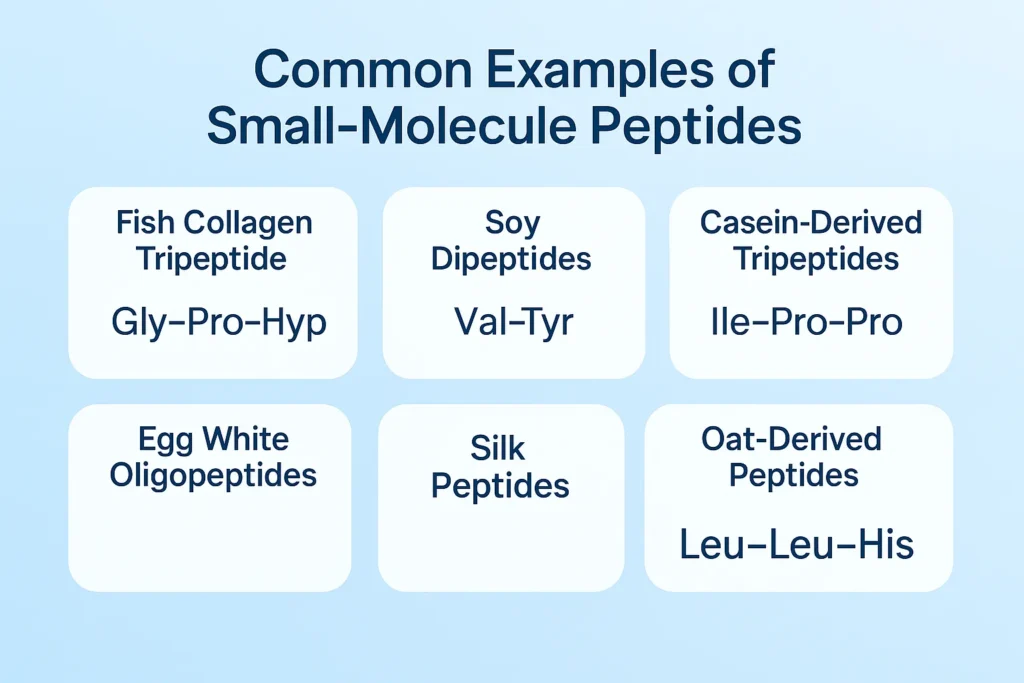
IV. Common Examples of Small-Molecule Peptides
Let’s look at several scientifically validated small-molecule peptides widely used in health and nutrition products:
1. Fish Collagen Tripeptide (Gly–Pro–Hyp)
Molecular weight: ~500 Da
Source: Enzymatically hydrolyzed fish collagen
Function: Promotes skin elasticity, reduces wrinkles, supports joint and bone health
Applications: Beauty-from-within supplements, anti-aging drinks
Note: Gly–Pro–Hyp is the most abundant repeating unit in collagen, and its tripeptide form is clinically shown to stimulate dermal fibroblast activity and collagen synthesis [4].
2. Soy Dipeptides (e.g., Lys–His, Val–Tyr)
Molecular weight: ~275–290 Da
Source: Hydrolyzed soy protein
Function: Antioxidant, antihypertensive, muscle support
Applications: Cardiovascular health products, sports recovery formulas
Benefit: Val–Tyr is a potent ACE inhibitor, supporting blood pressure control [5].
3. Casein-Derived Tripeptides (Ile–Pro–Pro and Val–Pro–Pro)
Molecular weight: ~310–330 Da
Source: Fermented or hydrolyzed milk casein
Function: Blood pressure regulation
Applications: Functional dairy drinks, nutraceuticals
Research: These tripeptides have been extensively studied for their ability to inhibit angiotensin-converting enzyme (ACE), helping reduce hypertension naturally [6].
4. Egg White Oligopeptides
Molecular weight: 200–1000 Da
Source: Enzymatically hydrolyzed ovalbumin
Function: Immune modulation, gut health, antioxidant activity
Applications: Immune-boosting supplements, functional beverages
Mechanism: Enhance intestinal barrier function and suppress inflammatory responses [7].
5. Silk Peptides
Molecular weight: ~300–600 Da
Source: Hydrolyzed silk fibroin
Function: Skin hydration, anti-aging, antioxidant
Applications: Skincare formulations, nutricosmetics, hair nutrition
Feature: Highly bioavailable and rich in glycine and alanine, which contribute to skin repair [8].
6. Oat-Derived Peptides (e.g., Leu–Leu–His)
Molecular weight: ~380 Da
Source: Hydrolyzed oat protein
Function: Gut health, cholesterol regulation, anti-inflammatory
Applications: Functional foods for heart and digestive health
Evidence: Shown to support gut barrier function and reduce LDL cholesterol in preclinical studies [9].
V. Production Technology: Enzymatic Hydrolysis
The development of enzymatic hydrolysis technology has revolutionized the production of bioactive peptides. This method mimics the body’s digestive process but in a controlled, scalable environment, producing peptide sequences with targeted biological effects and consistent molecular size profiles [10].
Key benefits include:
- Precision: Specific enzymes can generate di- and tri-peptides with desired functionalities.
- Purity: Minimizes allergenic or immunogenic fragments from parent proteins.
- Scalability: Industrial enzymatic hydrolysis allows for large-scale peptide production.
VI. Applications Across Health Categories
Small-molecule peptides are not confined to one health niche. Their versatility spans multiple applications:
| Category | Benefit | Example Peptide |
|---|---|---|
| Beauty-from-within | Improves skin elasticity & hydration | Fish Collagen Tripeptide |
| Cardiovascular Health | Blood pressure regulation | Val–Tyr, IPP |
| Gut Health | Enhances intestinal barrier | Oat Peptides |
| Immune Support | Modulates cytokine response | Egg Oligopeptides |
| Sports Nutrition | Muscle repair, antioxidant | Soy Dipeptides |
Their fast absorption and targeted activity make them ideal for modern consumers seeking efficient, science-backed health solutions.
VII. Conclusion: Small Peptides, Big Impact
In a world where bioefficacy and absorption matter more than ever, small-molecule peptides offer a powerful solution. Their scientifically proven benefits, enhanced absorption profile, and diverse applications make them stand out in the competitive landscape of functional ingredients.
For formulators, health brands, and end consumers alike, understanding the science of peptide size is critical. Whether it’s a 321 Da collagen tripeptide or a 280 Da soy dipeptide, the data is clear: when it comes to peptides, small is strong.
References
- Korhonen, H., & Pihlanto, A. (2006). Bioactive peptides: Production and functionality. International Dairy Journal, 16(9), 945–960. https://doi.org/10.1016/j.idairyj.2005.10.012
- Brandsch, M. (2013). Drug transport via the intestinal peptide transporter PepT1. Current Opinion in Pharmacology, 13(6), 881–887. https://doi.org/10.1016/j.coph.2013.09.008
- Udenigwe, C. C., & Aluko, R. E. (2012). Food protein-derived bioactive peptides: Production, processing, and potential health benefits. Journal of Food Science, 77(1), R11–R24. https://doi.org/10.1111/j.1750-3841.2011.02455.x
- Ohara, H., Matsumoto, H., Ito, K., Iwai, K., & Sato, K. (2007). Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. Journal of Agricultural and Food Chemistry, 55(4), 1532–1535.
- Miguel, M., López-Fandiño, R., Ramos, M., & Aleixandre, A. (2006). Short-term effect of egg-white hydrolysate products on the arterial blood pressure of hypertensive rats. British Journal of Nutrition, 96(2), 325–330.
- Seppo, L., Kerojoki, O., Suomalainen, T., & Korpela, R. (2002). The effect of a Lactobacillus fermented milk on hypertension: A placebo-controlled crossover study. Journal of Human Hypertension, 16(9), 625–630.
- Liu, J., Yu, Z., Zhao, W., Lin, S., & Wang, Y. (2018). Immunomodulatory and anti-inflammatory effects of egg protein-derived peptides: A review. Critical Reviews in Food Science and Nutrition, 58(13), 2071–2080.
- Zhang, Y. Q. (2002). Applications of natural silk protein sericin in biomaterials. Biotechnology Advances, 20(2), 91–100.
- Wang, C., & Liu, Y. (2019). Oat-derived bioactive peptides: A review of structure-function relationships and bioavailability. Journal of Functional Foods, 55, 221–230.
- Sarmadi, B. H., & Ismail, A. (2010). Antioxidative peptides from food proteins: A review. Peptides, 31(10), 1949–1956.
Frequently Asked Questions (FAQ)
Small-molecule peptides are short chains of amino acids, usually dipeptides or tripeptides, with a molecular weight under 1000 Daltons. Their small size allows for faster and more efficient absorption in the digestive system compared to larger proteins or polypeptides.
Molecular weight determines how peptides are absorbed in the body. Peptides under 1000 Da, especially those below 500 Da, are absorbed more efficiently via intestinal peptide transporters like PEPT1. This leads to higher bioavailability and quicker physiological responses.
Yes, when derived from food-grade proteins (such as fish, soy, milk, or eggs) and produced through controlled enzymatic hydrolysis, small peptides are generally recognized as safe (GRAS). Many have been used in clinical trials and food supplements with no adverse effects reported.
Collagen peptides are broader in size, often ranging from 1000–3000 Da, whereas collagen tripeptides (like Gly–Pro–Hyp) are specific sequences with a molecular weight around 300–350 Da. Tripeptides offer better absorption and targeted skin and joint health benefits.
Yes. Several small peptides, particularly fish collagen tripeptides, have been clinically shown to improve skin elasticity, hydration, and wrinkle reduction by stimulating dermal collagen synthesis from within.
Bioactive peptides are not found directly in foods but are released through hydrolysis (digestion or enzymatic treatment). The best protein sources for peptide extraction include:
Fish skin and scales (collagen peptides)
Soy protein (antioxidant and antihypertensive peptides)
Milk casein (blood pressure-regulating peptides)
Egg white (immunomodulatory peptides)
Oats (gut and cholesterol-regulating peptides)
Peptides, especially di- and tripeptides, are often absorbed more efficiently than free amino acids. In addition, many peptides have biological activities — such as reducing inflammation or oxidative stress — that free amino acids do not possess.
Look for products that:
Specify peptide size (e.g., <500 Da or tripeptide form)
Disclose source (fish, soy, egg, etc.)
Use enzymatic hydrolysis (not acid hydrolysis)
Provide clinical evidence or published studies
Are certified for purity and safety (e.g., ISO, HACCP, GMP)
Yes. Certain small peptides like branched-chain amino acid (BCAA)-containing dipeptides and antioxidant peptides from soy or egg can reduce muscle damage, enhance recovery, and modulate inflammation post-exercise.
While it varies by individual and application (e.g., skin, blood pressure, muscle), many users report visible or measurable benefits within 4–8 weeks of consistent, daily supplementation.